Introduction
It’s our pleasure to continue to provide this short article about vacuum measurement. Over the years it has helped many a budding expert get a first real handle on “how to measure nothing.” For more than 80 years Televac® has developed a line of diaphragm, thermocouple, convection, cold cathode, and hot ion sensors, along with highly reliable active gauge and controller instrumentation to provide user-friendly analog and digital outputs along with a visual display of vacuum readings. Televac® products combine state-of-the-art technology with well proven vacuum measurement techniques to provide the best vacuum measurement solutions on the market (at least we think so). Now that our sales pitch is out of the way, we hope you find the rest of our article enjoyable and informative.
Foreword: Don’t skip this…
Many scientific papers have been written on high-vacuum measurement – papers that can leave the industrial vacuum engineer somewhat flabbergasted. We’re hoping to change all that. This article was written by a layman, for layman, and in layman’s terms. It’s intended for you lucky people engaged in vacuum production processes who may not be too familiar with the bewildering array of terms to describe these processes – such terms as microns, thermal conductivity, residual gas, thermocouples, ionization… I know we could go on and on. These people are the future technicians who will perhaps, someday, maybe, buy their vacuum gauges from the fine company (there goes the sales pitch again) that gave them this masterpiece of literary achievement. If this article doesn’t help, we heartily recommend Vacuum Technology by Alexander Roth. Here you’ll find all the “meat” you’d probably ever care to digest.
Much ado about nothing, or, so you want to measure vacuum?
An article a while back in Chemical and Engineering News claimed in its title “Chemists are Lousy Speakers”. Among other observations, the article noted that all too often the technical papers presented at various symposia and meetings are completely void of any semblance of humor. The author, Carl J. Koenig, points to the saying: “A little nonsense now and then is relished by the wisest men.” He also quotes one of the proverbs for young engineers originated by Philip W. Swain, editor of Power: “To learn how to make a good speech, attend a typical engineering society meeting. Note just how the papers are presented. Then go thou and do otherwise.”
Koenig tells the story of hearing a scientist present a thesis on detecting leaks in a high-vacuum system. This scientist claimed they had spent hours and hours searching for a leak in a system and finally came to the conclusion that the best way to locate the leak would be to fill the entire system with sugar and observe where the ants crawled in. This bright suggestion lifted the cloud of boredom from the room and after a hearty laugh, everyone settled back to enjoy the further discourse on the subject of leakdetection.
Bearing this advice in mind, we have attempted to inject a note of humor throughout the pages of this article and we trust that it will relieve some of the monotony that is usually encountered by the layman when reviewing a technical subject. If you find you can’t detect the humor, call us and we’ll advise you where you can purchase the latest in Electronic Joke Detection instrumentation.
The controversies that have developed over the problem of measuring more and more of less and less have only been surpassed by the proverbial question of the chicken or the egg. This little treatise, therefore, should produce one of two effects: either it will answer many of the so-called “vacuum questionables” in your mind, or leave you with a questionable vacuum in your so-called mind. Everyone has heard the timeworn phrase, “Nature abhors a vacuum”. But that’s a very unfair statement because, within 500 miles of where you sit with this article, there exists a vast expanse of vacuum estimated to be about 10-10 Torr. That’s only 500 miles, straight up! Dr. Dushman of GE had an ingenious scheme for obtaining that wonderful vacuum here on Earth. “All you have to do,” said Doc, “is construct an airtight pipeline, straight up, and pipe the vacuum right into your plant.” Great idea Doc, but let’s get down to earth, and down to work.
Now before we set our feet on terra finna, we need to know where we want to stand. It’s sad but true that we often happily fall into our own little rut and fail to see or understand the other fellow’s viewpoint. So, it seems when we’re talking about vacuums. Joe Doaks talks about a vacuum of 27 inches, while his brother Oakie says it’s a vacuum of 76 millimeters. Along comes their cousin, Mairzy Doats, who calls it a vacuum of 76,000 microns, while Dosey says it’s a tenth of an atmosphere. Little Lamsey Divey joins the debate and says it’s really 76 Torr and when the International Organization for Standardization (ISO) has its way, it will be about 100 millibar or 10,000 pascals. Since they’re all relatives, an argument ensues – and continues down through the generations. Let’s see if we can determine the score and see who’s winning.
Let’s go back to school for a minute for a short session when you learned that the Earth’s atmosphere, under normal conditions at sea level, exerts a pressure of 15 pounds per square inch (that’s 14.696 lb/in2 for you hair splitters). We don’t feel this pressure because humans evolved so that we also exert 15 pounds per square inch internally, and thus the two pressures are equalized. But at high altitudes, nosebleeds are common because our internal pressure exceeds the external atmospheric pressure and if we go too high, we’re liable to blow up.
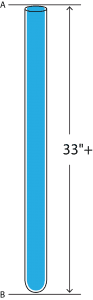 Since we’re in school, we will now conduct an experiment. Please look at the glass tube shown to the left. We’ll assume that the length of the tube (AB) is greater than 30”. Now we will pour mercury into the open end and fill the entire tube. Then, carefully holding the index finger of your right hand over the open end, turn the tube upside down
Since we’re in school, we will now conduct an experiment. Please look at the glass tube shown to the left. We’ll assume that the length of the tube (AB) is greater than 30”. Now we will pour mercury into the open end and fill the entire tube. Then, carefully holding the index finger of your right hand over the open end, turn the tube upside down
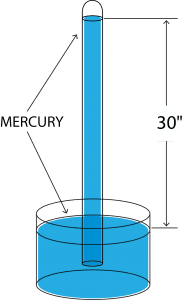
to an inverted vertical position and insert it into a reservoir containing mercury as shown to the right. Place the open end under the surface of the mercury in the reservoir (or well). Remove your finger.
Will the mercury run out all over the floor? No, because the atmospheric pressure exerted on the mercury in the well will support a column of mercury 30” high. This is known as a “well-type” mercury manometer. Now here’s where we get technical. Actually, under normal conditions the supported column will be 29.92126 inches, but 30” is
close enough for when you’re measuring vacuum with a yardstick. We also don’t suggest trying this experiment at home – mercury is very bad for you and for the environment.
With our experimental set-up, the atmosphere is exerting a pressure upon the surface of the mercury in the well that supports the 30” column. Now, if this entire apparatus were enclosed in an airtight chamber and we started to pump the air out, it would naturally reduce the pressure on the surface of the mercury in the well – thus the column of mercury in the tube would begin to fall.
That’s why, when the weatherman’s mercury column, or barometer as he calls it, falls to 27”, you’d better batten down the hatches and bring in the cat, because you’re in for a blow. It means that a vacuum of 3” has been created in your vicinity and the surrounding air from the higher-pressure areas will rush in where others would fear to tread.
Here’s where an explanation of the controversy concerning the terminology might come in handy. If we have a vacuum of 3”, it means that the 30” column will be lowered 3” and leave a column of mercury 27” high. Actually, in terms of real vacuum technology, it’s not a vacuum at all. Instruments known as “draft gauges” have been developed that measure “vacuums” that fall within this category. They’re used to measure the draft or suction created by such phenomena as a rising column of air. These gauges are useful in the measurement of the draft in smokestacks and are used to help determine the efficiency of furnace combustion as well as other processes.
Now, a lot of people are interested in saving money, and mercury, and are not particularly worried about duplicating the accuracy of the 30” straight tube. In order to put these frugal tendencies into practice, the well-known mercury U-tube was brought into being.
Here, for every 1” change in vacuum, the column of mercury on the right side of the U-tube rises ½” and the column on the left side Iowers ½”, with the result that the levels of mercury in the two columns are brought 1” closer to each other. Thus, a “perfect” vacuum will be indicated when the mercury in the right column has been raised 15” and mercury in the left column has been lowered 15” so that the levels of the two columns are equal. This is a 30” vacuum.
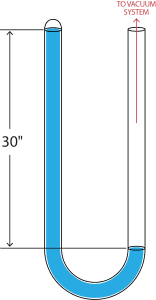
The U-tube still requires a tube 30” high. If the engineer is primarily interested in the better vacuum, a shorter U-tube can be used, which will come on scale only when a vacuum indicated by the upperlimit of the gauge has been reached. Thus, a U-tube 6” high will not come on scale until a vacuum of 24” is obtained. Before then, the engineer can only hope that the vacuum pumps are working satisfactorily.
With this pocket-sized edition of the mercury column, we also run into a confusion over terminology. For example, “What is a 3” vacuum, is it 3” from absolute zero or 3” from atmospheric zero?” If you stay with us, we’ll endeavor to clear up this mess.
Some people aren’t bothered by size, and to obtain increased accuracy they substitute oil for mercury, since oil has a known specific gravity. Thus, it is possible to increase the length of the 30” column and obtain more accurate readings. If we used oil, weighing 1/10th as much as mercury, atmospheric pressure would support the equivalent of ten 30” columns – or a single column 300” high. Then a change of 1”, as measured by a mercury column, would be indicated by a change of 10” on the oil manometer.
Where minute changes in draft are encountered, it is advisable to refer to the vacuum in terms of inches of water rather than inches of mercury. A change of 1” in a water column is equivalent to a 1” change of approximately 0.07” in a 30” mercury column. Therefore, the vacuum terminology “inches of water” is merely a refinement or magnification of “inches of mercury”.
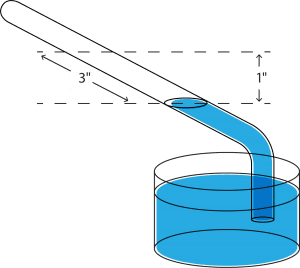
To obtain increased accuracy, the tube is usually mounted on an inclined plane, as shown in the sketch to the right. Note that, in using this system, a 1” change in vacuum would be extended over a 3” length of the inclined tube.
Using this method, accuracies on the order of 0.01” of water (that is 0.0007” of mercury) may be obtained, thus providing very accurate indications of small changes. Don’t forget, however, that mercury manometers are also used for measuring relatively high vacuums, because they are adaptable over the entire range from 0” to 30”.
But we ain’t seen nothin’ yet – since a vacuum in terms of a few inches of water really isn’t a vacuum, let’s move down the scale a bit closer to nothin’. We mentioned above that a 3” vacuum (-3” gauge pressure) would lower the 30” column of mercury a net 3” and that therefore the mercury column would stand 27” high. Following from that, we can readily understand that if the vacuum continues to improve until the pressure exerted on the surface of the mercury in the well will no longer support any mercury in the column, we have reached a “high vacuum” of 30” (-30” gauge pressure).
It is in this range of 30” (or 0 Torr) that most controversies over vacuum terminology occur. Let’s take it easy and bleed a little air into our 30” vacuum chamber and raise the mercury column ½” so that we now have a 29 ½” vacuum. This is considered “rough vacuum” or “high vacuum” in many industrial applications. We’ll prove later that we still ain’t seen nothin’ yet.
In the 29” to 30” range of vacuum, we usually switch terminology to the metric system. Then, measuring the ½” from the absolute zero line (“perfect” vacuum), we find that our 29 ½” vacuum is also a pressure (or vacuum) of 12.7 mm (1/2” = 12.7 mm). Note that the scale is now inverted and someone is likely standing on their head. In terms of inches of mercury, 0 usually means atmospheric pressure (zero vacuum) and 30” is a perfect vacuum (-30” gauge pressure).
But in terms of millimeters, 0 pressure is the perfect vacuum (absolute zero), and all measurements are made from this reference point. Atmospheric pressure, of course, is 760 mm (+29.92126” absolute pressure), and therefore 76mm equals 1/10th of an atmosphere (Remember our ol’ pal Dosey Doats?) Let’s be content with the fact that, in our particular case, we have a 29 ½” vacuum in terms of mercury and at the same time we have a 12.7 mm vacuum in terms of millimeters of mercury. Some, reluctant to use the metric system, will stand on their heads and argue that we also have a 1/2” vacuum. Unfortunately, they are also correct.
Most of the industry has accepted the term “Torr” (honoring Torricelli) for millimeters of mercury. Micron (a thousandth of a millimeter) is thus expressed as milliTorr. As before mentioned, lest this treatise be come obsolete before it is widely circulated, we must mention that a totally different scale may reluctantly (at least by us) be adopted that may be called millibar or Pascal (recognizing another long-gone scientist). This latter has about as much relationship to millimeter or Torr as the meter has to the yard. Just in case you’re curious about what’s coming up, 30” = 760 mm = 760 Torr; soon you’ll see it’s also equal to 1 bar or 1,000 millibars. Hang in there!
Sometimes vacuum engineers are blinded by their own narrow categories and practices and so can’t fathom what the other fellow is talking about. Are you still with us? If not, see the photograph on the next page, it should clear things up.
 |
 |
Since you’re now all straightened out about vacuum in terms of inches and millimeters of mercury and Torr, we will transfer you to the micron department, where you can more nearly comprehend the true meaning of nothing. Before we actually enter this realm though, it might help to mention some other types of gauges that are conventionally used to indicate vacuums within the range of 0” to 30”.
Many objects to the 30” glass manometer tubes – their fragility and the presence of mercury makes some people uncomfortable. Also, Big Brother in Washington – OSHA – views the presence of mercury in a manufacturing environment as a hazard. Sufficient accuracy for many purposes can be obtained by using a more rugged instrument that indicates the vacuum on a dial. These dial-type instruments operate using any one of these different systems; the Bourdon tube, the bellows, or the diaphragm. All of these devices utilize the same basic principle that a changing pressure expands, contracts, twists or otherwise distorts the measuring element, which is then linked to an indicating needle that sweeps the dial. The main disadvantage of this type of gauge is that changes in atmospheric pressure and temperature act as “bugs” in the system – they must be compensated for to maintain accurate indications.
There’s another “bug” – hysteresis. That’s the tendency for any material not to return to its original shape or position after it has been distorted. Unless the material used in the construction of the actuating elements and mechanisms are carefully chosen, a distortion will gradually develop and you’ll end up with an erroneous reading. Before you know it – hysteresis!
The problems raised by atmospheric pressure changes can be overcome rather easily by evacuating the sensitive measuring elements to a high vacuum. Thus any increase in pressure above absolute zero would give the desired indication or movement of the element. Gauges of this type, however, are sensitive to overpressures, and a sudden accidental surge of pressure above atmosphere is likely to cause damage.
Thanks to modern microprocessor-based electronics, a diaphragm-type gauge has been developed that serves a range down to 1 Torr (or 1 mm of mercury) quite well. (I feel another sales pitch coming on). What’s more, it does this with an accuracy of ±1 Torr, and takes overpressure up to 30 psi. Incidentally, we call this little miracle of vacuum measurement technology the Televac® 1E Piezo Diaphragm Vacuum Gauge.
Let’s see now … where were we? – Oh, yes! – We were about to graduate you to the micron department. We had explained This confusion reminds me of the story of the four blind men and the elephant. When they were introduced to Jumbo, all reached out to feel the animal and determine what it was like. One grabbed the elephant’s leg and argued that the elephant was just like a tree. Another put his mitts on the elephant’s trunk and argued that the animal was like a snake. The third man happened to be standing by the elephant’s side and when he felt the expanse of flat area he naturally argued that the elephant was similar to a wall (stucco, of course). The fourth fellow got a hold of that portion of the anatomy usually held on the south end of a north-bound elephant (the tail), and argued that the elephant was like a rope. All four were right, but they couldn’t seem to get together. that the term “inches of mercury” was for those who use a yardstick to measure their vacuum. The term is usually applicable to vacuums over the entire range of 0” to 30”. “Millimeters of mercury” or “Torr” is simply a refinement of this measurement similar to the refinement of a pair of calipers over the yardstick.
 “Micron” designates an even finer measurement than Torr and uses (theoretically, of course) an electron microscope. 1 Torr is equal to 1/25th of an inch and a micron is equal to 1/1,000th of a Torr. Try to imagine a comparison between the planet, the Empire State Building, and an ant, or maybe just his little toe (of course ants don’t have toes… so you’ll just have to work with me). Since we measure vacuums down to 1/100,000th of 1 micron (0.00001 micron) or 10-8 Torr, you can see that we are rapidly approaching “nothing” – but we’re not there yet.
“Micron” designates an even finer measurement than Torr and uses (theoretically, of course) an electron microscope. 1 Torr is equal to 1/25th of an inch and a micron is equal to 1/1,000th of a Torr. Try to imagine a comparison between the planet, the Empire State Building, and an ant, or maybe just his little toe (of course ants don’t have toes… so you’ll just have to work with me). Since we measure vacuums down to 1/100,000th of 1 micron (0.00001 micron) or 10-8 Torr, you can see that we are rapidly approaching “nothing” – but we’re not there yet.
Just to refresh your memory, 10-8 Torr is merely a convenient way of saying 0.00000001 Torr. An easy way to remember the conversion is to count how many places you have to move the decimal point. For example, 0.001 would be 1*10-3. If all of these relationships are still confusing, take a gander at the Vacuum Measurement Conversion Tool on our website. Now that you have been formally introduced to the micron, you can consider yourself part of a select few and can be dubbed Chief Potentate of Punk Pressure.
From here on in, your main problems will be concerned with the ways of measuring vacuum in terms of Torr and micron. Don’t forget that a 12.7 Torr vacuum is equivalent to 12,700 microns. As mentioned above, our literary endeavor will reveal ways and means of measuring vacuums down to 0.00001µ or 0.00000001 Torr or 10-8 mm Hg (Hg is someone’s idea of chemically expressing the element mercury. They figured it would save ink. And “µ” by the way, is the ink saver for micron.)
To those of you who have thus far been measuring vacuum with a ruler, these figures will seem something like the national debt in reverse and thus won’t mean very much, but at least we have agreed on our terminology. We can now freely discuss the measurement of high vacuum with those of you at the head of the class.

Diaphragm Gauge
This gauge measures the movement of a flexible diaphragm caused by an applied pressure. It utilizes various techniques to measure this deflection. One technique measures the change in capacitance between a fixed electrode and a moving diaphragm (called a capacitance diaphragm gauge). A second technique uses the signal from a strain gauge directly attached to the diaphragm. Diaphragm gauges are typically used to measure pressures between 760 Torr and 0.001 Torr (or from 1 Atm to approximately 1/1,000,000 of an Atm). However, by using multiple transducers, it is possible to extend this range such that pressure below 10-5 Torr can be read.
This gauge is not sensitive to gas type. These sensors are temperature compensated, but do respond to rapid changes to temperature. Temperature changes may cause zero shifts. Users should note that occasional adjustments of the zero point are normal for sustaining accuracy with this type of sensor, and provision for making this adjustment should be included in the installation.
Thermal Conductivity Gauges
There are two general types of gauges on the market in this general classification of vacuum gauges. One is known as the Pirani gauge and the other is known as a thermocouple gauge. Both types operate on the thermal conductivity principle, which we will now undertake to explain. These gauges measure the thermal conductivity of the residual gas. The ability of the gas in the vacuum system to conduct the heat away from the filament (or “hot wire”) is a function of the thermal conductivity of the gas. These gauges measure this, and so are also known as “hot wire gauges”.
To measure vacuum by the thermal conductivity method, we apply a constant voltage and current to a filament mounted within a gauge connected to the vacuum system. The temperature of the filament will eventually assume equilibrium. The heat generated by the filament is being conducted away from the immediate vicinity of the filament by the molecules of the gas surrounding it. As we start to pump the gas out of the vacuum gauge, the wire becomes hotter because there are fewer molecules of gas present to conduct the heat away from the filament.
The more the gauge is evacuated, the hotter the filament becomes with the same applied voltage and current. Eventually, as we reach a high vacuum (on the order to 0.1 micron), we can assume that the filament has reached its maximum temperature because there are relatively few molecules of gas left within the gauge to conduct the heat away. Actually, there are still billions of them present, but they’re like the American dollar – they don’t mean very much because they’re so small.
 The difference between the Pirani gauge and the thermocouple gauge is the method used to measure the changing temperature of the filament. The Pirani gauge generally uses a single filament; as the temperature of the filament increases, its resistance also increases and the temperature of the wire is actually measured in terms of resistance. The gauge can then be calibrated with an accurate capacitance diaphragm gauge, using dry air (or any other dry gas for which you wish to calibrate the gauge). Naturally, the gauge can be calibrated directly in microns after the various points on the scale are established – a given temperature (or resistance) equaling a given vacuum as indicated by the capacitance diaphragm gauge.
The difference between the Pirani gauge and the thermocouple gauge is the method used to measure the changing temperature of the filament. The Pirani gauge generally uses a single filament; as the temperature of the filament increases, its resistance also increases and the temperature of the wire is actually measured in terms of resistance. The gauge can then be calibrated with an accurate capacitance diaphragm gauge, using dry air (or any other dry gas for which you wish to calibrate the gauge). Naturally, the gauge can be calibrated directly in microns after the various points on the scale are established – a given temperature (or resistance) equaling a given vacuum as indicated by the capacitance diaphragm gauge.
With the thermocouple gauge, the same principle is involved, except that in this case the temperature of the filament is measured instead of the resistance. This accomplished by means of a thermocouple welded to the filament wire. The thermocouple naturally assumes the same temperature as the filament, and a definite potential is developed. This potential in millivolts, can then be calibrated directly in microns. (If you know what thermocouple or potential means – don’t bother reading the next section).
Thermocouple Gauge
Very often such terms as thermocouple and potential are tossed around so promiscuously by those “in the know” that Junior just watches them go by. A thermocouple is merely a couple of wires made of different materials joined together. Any two different metals will do, though some combinations work better than others. The “black magic” starts when these two wires are joined together. Just because they’re different, they start generating voltage, or potential, as it will now to called. The unique thing about a thermocouple, however, is that when it’s subjected to heat (or any other change in temperature), the millivoltage generated by these two different metals changes proportionately – wonderful, isn’t it?
As the thermocouple gets hotter, it generates more millivolts, and these changing potentials can easily be measured. All you have to do is calibrate the changing temperature of the hot wire in terms of millivolts generated by the thermocouple and you have a thermocouple-type vacuum gauge. Now the capacitance diaphragm gauge comes into play again, and you have to know which potential equals which level of vacuum.
With thermocouple-type gauges, the filament current and voltage must carefully be maintained to ensure a stable calibration. It’s also necessary that the filaments not become dull or tarnished from the condensation of any contaminating vapors present within the vacuum system. As soon as the wires become slightly dull, a radiation loss is seen; the radiation loss affects the calibration of the gauge.
To overcome this difficulty, the company that keeps my family in food and clothes (you will find their name on the back cover) has devised a unique method of protecting the filaments. Our founder theorized that if the filaments were precoated before the calibration of the gauge, that calibration would not be affected by additional contamination caused by the vapors in the vacuum system. This method was introduced in the Televac® thermocouple gauges. (Yet another sales pitch, the temptation was just too great!)
The gauges can be constructed in one of two ways, the first method used is a reference gauge (or standard) that has been evacuated to a vacuum of 0.1 micron and is hermetically sealed. The same current and voltage that is applied to the wires in the measuring gauge (the gauge that is connected to your vacuum system) is also applied to the wires within the sealed reference tube.
The temperature of the two heater wires is then compared and the difference is used as a basis for determining the vacuum. As the vacuum in the measuring tube approaches the vacuum in the reference tube, the temperature of the hot wire approaches equality. The use of the reference tube permits compensation for ambient temperature changes because the two cells will always be at the same temperature. Thus, an equalizing effect is introduced in the event abnormal changes take place in the surrounding temperatures.
The second method of employing the thermal conductivity principle ignores the use of the reference tube and merely measures the temperature of the filament in the measuring tube. Ambient temperature compensation is accomplished through the use of a negative temperature coefficient resistor (NTC, also sometimes called a thermistor) in the metering circuit.
Unless special circuitry is employed to increase the signal at higher pressures, the hot-wire gauges are limited in their range to an upper limit of about 20,000 microns (for a range of 10-3 to 20 Torr). Accuracy above the 500-micron point is somewhat limited because the change in the thermal conductivity of the residual gas between 500 and 20,000 microns is very small. Although developments have extended this range considerably, it’s still very difficult to make an element sensitive enough to accurately determine those minute differences in thermal conductivity. Limitations also hold true on the lower end of the scale – below 1 micron, changes in thermal conductivity are very minute.
Another problem that confounds the user of the hot-wire type gauges is the fact that gases other than the one for which the gauge was originally calibrated can affect the accuracy of the instrument. For instance, dry air has a thermal conductivity of 1, but hydrogen, on the other hand, has a thermal conductivity much greater than that of air. So if the gauge were subjected to an atmosphere of hydrogen, about 5 times as much heat would be conducted away from the filament as would be conducted by air at the same pressure. Therefore, the filament would be cooler at the same pressure and so would give an erroneous indication of poorer vacuum (or higher pressure).
Hydrogen, of course, is an extreme example. Most vacuum systems do not contain an excess of these high-conductivity gases. However, other gases – such as CO2, water vapor, nitrogen, alcohol, mercury, and oil vapors – which produce a slight effect on the calibration of the gauge, are very often present.
The thermal conductivity of various combinations of these gases, although not identical to that of air, is otherwise close enough so that the industrial vacuum engineer can duplicate his readings and establish this cycling process with relatively little trouble using the hot-wire gauge. It’s safe to say that many vacuum processes are primarily concerned with pumping air or nitrogen from a given chamber; therefore, the calibration of the gauge can be considered quite accurate.
Convection Gauge
The convection gauge sensing element consists of a pair of wires heated by the passage of a current which maintains a constant temperature. Thermocouples are welded to the center of the wires there by providing a means of directly measuring the temperature.
To maintain a constant temperature, the current is increased as the pressure in the sensor is increased since more air is available to cool the heated wire.
The response of the sensor depends on the gas type. These sensors are compensated for room temperature variation and are calibrated for operation in the vertical position. The range of these sensors is from 10-3 to 103 Torr. Zero and atmosphere (atmospheric pressure) calibration points are provided for occasional adjustments as required.
Ionization Gauge
The gauges thus far discussed cover many of the useful ranges encountered in vacuum processes today, but we still have not considered the “high” vacuum department, which requires accurate measurement down to 10-10 or 0.0000001 microns. (That’s one one-hundred millionth of a Torr in case you want to measure it with a ruler). For this purpose, the ionization gauge is usually employed and, with a properly constructed gauge, the scientist can measure vacuum as low as 1*10-11 Torr. (If you’ve measured vacuums in this range, you are a scientist).
The highest pressure to which the hot cathode ionization gauge can be continuously subjected is on the order of 1 micron, because pressures above this subject the filament to an oxidizing effect that causes frequent burnouts and encourages bad language. Thus the hot cathode ionization is adapted primarily to those processes that now require an ultra-high vacuum well below 1 micron.
Hot Cathode Ionization Gauge
Let us discuss the principal behind the hot cathode ionization gauge in relation to our diagram below featuring Joe Electron a tungsten filament within the gauge attached to your vacuum system is heated to incandescence and emits negatively charged electrons like Joe. Never mind how or why – it just happens. After these electrons are emitted from the filament, they travel in a straight line bound for no-one-knows-where and come in contact and collide with molecules of air that happen to be left in the system.
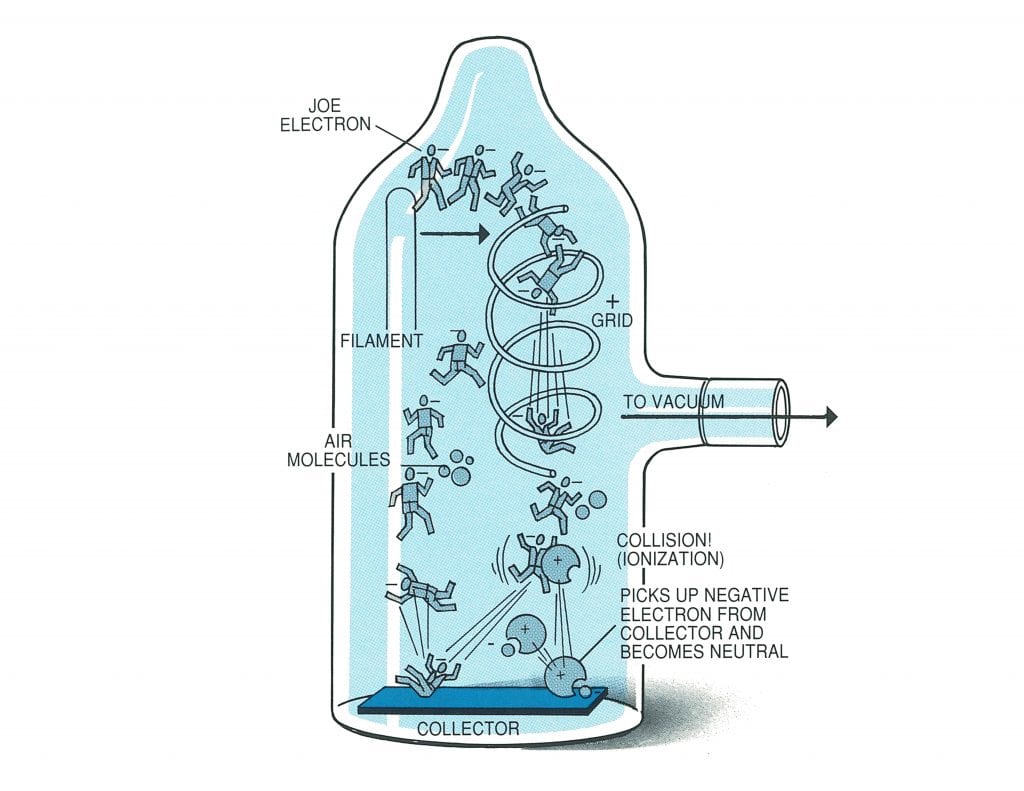 Bayard and Alpert found out that if a spiral of wire, called a grid (it looks sort of like a circular staircase) was constructed next to the filament and charged positively with electricity, the traveling negative electrons from the filament were given a “shot in the arm” and sped up in their journey through space. This speed-up process (acceleration) is caused by the tremendous attraction that the negative electrons have for the positive grid; the grid acts somewhat as a magnet. However, there are so many of these electrons coming from the filament that the majority of them fail to put on the brakes as they approach the grid. Instead, they whiz past it toward a metal wire that has been placed inside the grid. This metal wire is called the “collector” and is charged negatively.
Bayard and Alpert found out that if a spiral of wire, called a grid (it looks sort of like a circular staircase) was constructed next to the filament and charged positively with electricity, the traveling negative electrons from the filament were given a “shot in the arm” and sped up in their journey through space. This speed-up process (acceleration) is caused by the tremendous attraction that the negative electrons have for the positive grid; the grid acts somewhat as a magnet. However, there are so many of these electrons coming from the filament that the majority of them fail to put on the brakes as they approach the grid. Instead, they whiz past it toward a metal wire that has been placed inside the grid. This metal wire is called the “collector” and is charged negatively.
Well, Joe Electron has now whizzed through the gird, receiving a kick in the pants as he goes through, and is traveling at a high speed toward the collector. The molecules of air up ‘til now have been neutral, and the positive protons just balance the negative electrons that combine to make up the molecule. As the electron from the filament speeds towards the collector, a molecule of air gets in his way and – Wham! They collide!
When that happens, negatively charge Joe Electron knocks a negative electron from the molecule of air, and the molecule now becomes positively charged (because it is minus one negative electron). The process is known as ionization. Joe Electron then continues on his journey (a hit-and-run driver no less); as he approaches the negatively charged collector, he is repelled because he is also negative.
Joe turns around and heads back to the positively charged grid, and eventually winds up being hurled through the spiral grid again. The positively charged molecule of air, minus one negative electron, heads for the negatively charged collector and is received with open arms. The collector feels sorry for the poor molecule minus one electron, so it, in turn, gives up one of its own negative electrons and so restores the molecule to its original neutral status. It is this flow of electrons from the collector that we measure (in terms of microamperes) to give us the degree of vacuum. The number of molecules of air in the vacuum gauge is in direct proportion to the ionization that takes place, and therefore, in direct proportion to the flow of electrons given up by the collector.
Ergo, the better the vacuum, the fewer the molecules of air present, and the fewer the collisions, and the fewer the electrons that flow from the collector. In fact, at very low pressures, on the order of 10-11 Torr, special equipment is needed to amplify and measure the collector current. This condition makes it a swell day for Sunday drivers and even Joe’s wife, Jane Electron, who aspires to be a hit-and-run driver like her husband, can’t find very many air molecules to hit. If you could throw a stone straight out into space, and it continues to travel on and on in a straight line, your chances of hitting a star would be pretty slim, even though there are billions of stars to aim for. Similarly, the air molecules are so small that although there are millions present in a high vacuum, the space between them is so vast that collisions are few and far between. Thus in your high-vacuum set-up, we have a subminiature version of the universe.
One of the problems encountered when calibrating the ionization gauge is that the filament current must be controlled so that a constant flow of electrons is emitted from it. The positive grid must also remain sufficiently charged so that the speed of the electrons is not impaired. If this charge becomes weak, the negative electrons from the filament will not obtain enough speed (force) to ionize the air molecules.
Another disadvantage of this type of gauge is that the filament will be damaged if the pressure rises to about 10 microns, or if the gauge is subjected to continuous use above 1 or 2 microns. Because of accidents, or some other cause, the filament will immediately burn out. You remember what a tough time ol’ Tom Edison had trying to keep a filament glowing at atmospheric pressure. Somehow or other the filaments didn’t hold up. When he evacuated the bulb – presto! Electric bulbs were born.
An interesting feature of the Televac® ionization gauge, which appeals especially to the industrial users, is that the filament of the Televac® gauge cannot burn out as a result of accidents or other causes that bring about a sudden increase in pressure. As soon as the pressure of the vacuum system rises above 1 or 2 microns, the current to the filament is instantly and automatically cut off. That saves the filament and prevents costly shutdowns and frequent gauge replacements. It also saves wear and tear on the nerves and temper.
Cold Cathode Ionization Gauge
The hot filament (or hot cathode) ionization gauge is the accepted standard in the laboratory for measuring “high” vacuums, but it has drawbacks for industrial use. The gauge tubes are usually either of delicate glass construction or are “nude” tubes, which consist of unprotected elements projecting into the vacuum chamber. Both such “sensing heads” are subject to damage or contamination from the system, which renders them useless.
Thus, another type of ionization gauge has found favor in recent years for industrial environments such as vacuum furnaces and electron beam welders. This is a cold cathode gauge, of which there are several types including the Penning gauge and double inverted magnetron gauge. Two parallel connecting cathodes are used and the anode is placed midway between them. The cathodes are metal plates or shaped metal bosses; the anode is a loop of flattened metal wire, the plane of which is parallel to that of the cathode. A potential difference of 2 kV to 4 kV is maintained between the anode and the cathodes. In addition, a magnetic field is applied between the cathodes by a permanent magnet, which is usually external to the gauge tube body.
Electrons emitted from either of the cathodes travel in helical paths (due to the magnetic field), eventually reaching the anode, which carries a high positive charge. During the journey down the long electron path, many of the electrons collide with the molecules of residual gas, creating positive ions that travel directly to the cathodes. The ionization current that is thereby produced is read out directly on a micro-ammeter in terms of pressure.
The reliable pressure range of this instrument is usually 10-3 to 10-8 Torr although newer designs have extended that range both upwards and downwards. The upper limit is set by the glow discharge that appears, and can vary somewhat by the composition of the residual gas or the cleanliness of the gauge tube elements. The lower limit is set by the smallest ion current that can practically be measured, and the seals. The advantage of this type of ionization gauge is that the cold cathode gauge is very rugged and can be easily disassembled for cleaning. The instrument can be cleaned by means of glass bead abrasive blasting of the elements or using a simple abrasive pad.
In Closing…
There are many good and acceptable gauges on the market; I guess all of them have their advantages and disadvantages. It remains for some genius to devise an all-purpose vacuum gauge that could be used in the laboratory and in industry; that will maintain a stable calibration in spite of all the boneheaded mistakes the human elements can come up with; that can withstand sudden surges of pressure without fail; that will be unharmed when filled with vacuum pump oil that has backed up into the system after Junior forgets to release the vacuum; that will be unaffected by temperature and atmosphere conditions or the presence of stray and sometimes corrosive gases; that can be joggled, tapped, and sworn at by engineers who think the reading is wrong; that can be adapted for all vacuum ranges between 10-11 Torr and atmospheric pressure – and that will sell for only a few bucks.
If any genius reading this can devise such an instrument, begin filling out your retirement papers now! As you can see, there are still many problems involved in measuring vacuum. Hundreds of them aren’t even mentioned in this booklet; even if they were, you have probably encountered a couple of new ones. All we can say is that high-vacuum processing is indeed fascinating, and that we are standing on the threshold of a new era in industrial development employing vacuum procedures. Many new problems will be encountered; it’s up to the high-vacuum engineer to meet those challenges with an open mind. New ideas are needed, and the industry is looking to you.
– J. Gordon Seiter
As respectfully updated by
John J. (Jack) Boericke, 1976
William H. (Bill) Bayles, 1993
Shawn Orr and Jonathan Lance, 2019





